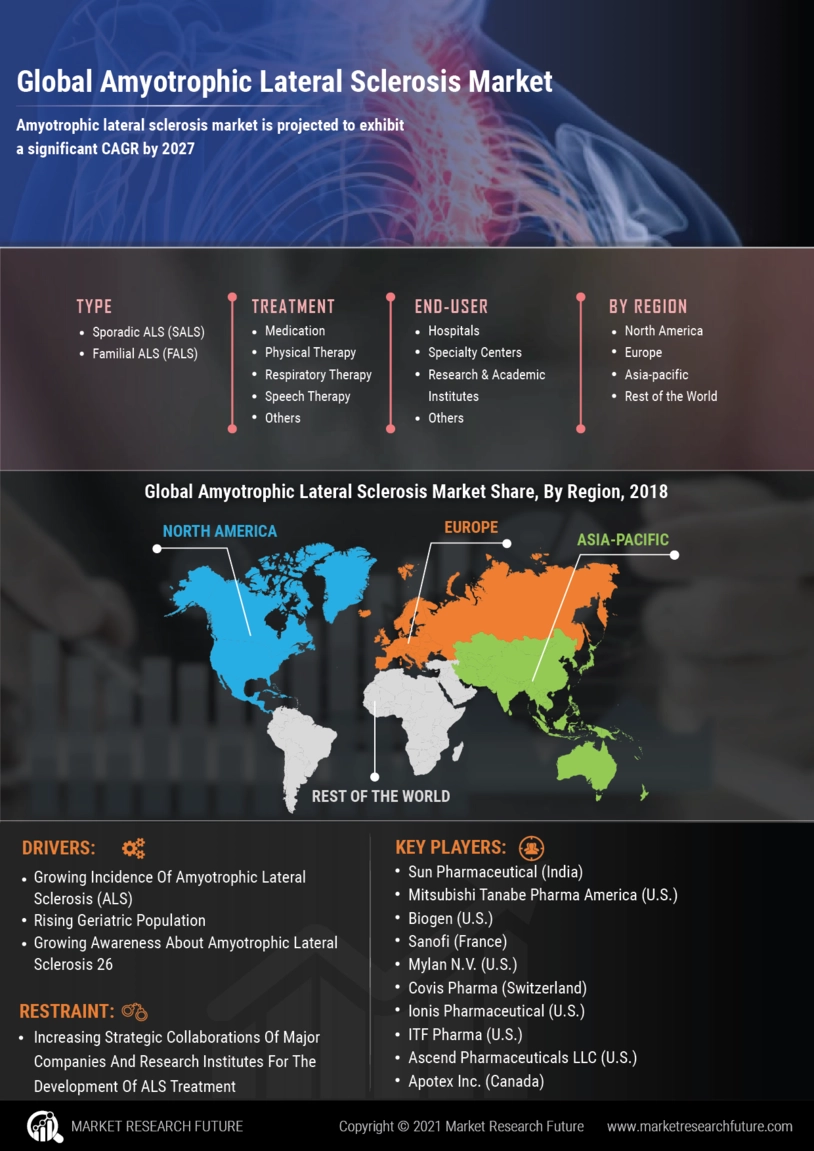
Amyotrophic Lateral Sclerosis Market: Key Factors Driving Market Growth
The Amyotrophic Lateral Sclerosis Market is experiencing gradual expansion as demand for advanced neurological treatments continues to rise. ALS is a rare yet severe condition that requires long-term medical intervention, creating sustained demand for effective drugs and supportive therapies. Increasing diagnosis rates and improved disease awareness are major contributors to market growth.
Pharmaceutical companies are focusing on developing innovative therapies that address the underlying mechanisms of ALS. These efforts include gene therapy, RNA-based treatments, and neuroprotective agents. Based on recent Amyotrophic Lateral Sclerosis market growth
observations, investment in research pipelines is a key driver shaping the competitive landscape. Companies that successfully advance clinical candidates gain significant strategic advantages.
Another important growth factor is the availability of supportive regulatory frameworks. Orphan drug designations and accelerated approval programs enable faster market entry for ALS therapies. These policies not only encourage innovation but also improve patient access to new treatment options. As a result, manufacturers are increasingly prioritizing ALS within their rare disease portfolios.
Healthcare infrastructure improvements also support market growth. Advanced diagnostic tools allow earlier detection, while specialized care centers enhance treatment delivery. Additionally, expanding reimbursement coverage in certain regions improves affordability and access to therapies, further stimulating demand.
Get Full Reports:https://www.marketresearchfuture.com/reports/amyotrophic-lateral-sclerosis-market-5822
Technology adoption is strengthening market expansion as well. Digital platforms enable better patient monitoring, while data-driven research improves treatment personalization. These advancements help optimize care outcomes and improve long-term treatment adherence.
In summary, the Amyotrophic Lateral Sclerosis Market is driven by scientific progress, regulatory incentives, and growing healthcare capabilities. These combined factors create a stable foundation for continued market development.
FAQ
Q1: What is the main driver of ALS market growth?
A1: Increased R&D investment and regulatory support for rare diseases.
Q2: Does early diagnosis impact market growth?
A2: Yes, early diagnosis increases treatment demand and duration.
The Amyotrophic Lateral Sclerosis Market is experiencing gradual expansion as demand for advanced neurological treatments continues to rise. ALS is a rare yet severe condition that requires long-term medical intervention, creating sustained demand for effective drugs and supportive therapies. Increasing diagnosis rates and improved disease awareness are major contributors to market growth.
Pharmaceutical companies are focusing on developing innovative therapies that address the underlying mechanisms of ALS. These efforts include gene therapy, RNA-based treatments, and neuroprotective agents. Based on recent Amyotrophic Lateral Sclerosis market growth
observations, investment in research pipelines is a key driver shaping the competitive landscape. Companies that successfully advance clinical candidates gain significant strategic advantages.
Another important growth factor is the availability of supportive regulatory frameworks. Orphan drug designations and accelerated approval programs enable faster market entry for ALS therapies. These policies not only encourage innovation but also improve patient access to new treatment options. As a result, manufacturers are increasingly prioritizing ALS within their rare disease portfolios.
Healthcare infrastructure improvements also support market growth. Advanced diagnostic tools allow earlier detection, while specialized care centers enhance treatment delivery. Additionally, expanding reimbursement coverage in certain regions improves affordability and access to therapies, further stimulating demand.
Get Full Reports:https://www.marketresearchfuture.com/reports/amyotrophic-lateral-sclerosis-market-5822
Technology adoption is strengthening market expansion as well. Digital platforms enable better patient monitoring, while data-driven research improves treatment personalization. These advancements help optimize care outcomes and improve long-term treatment adherence.
In summary, the Amyotrophic Lateral Sclerosis Market is driven by scientific progress, regulatory incentives, and growing healthcare capabilities. These combined factors create a stable foundation for continued market development.
FAQ
Q1: What is the main driver of ALS market growth?
A1: Increased R&D investment and regulatory support for rare diseases.
Q2: Does early diagnosis impact market growth?
A2: Yes, early diagnosis increases treatment demand and duration.
Amyotrophic Lateral Sclerosis Market: Key Factors Driving Market Growth
The Amyotrophic Lateral Sclerosis Market is experiencing gradual expansion as demand for advanced neurological treatments continues to rise. ALS is a rare yet severe condition that requires long-term medical intervention, creating sustained demand for effective drugs and supportive therapies. Increasing diagnosis rates and improved disease awareness are major contributors to market growth.
Pharmaceutical companies are focusing on developing innovative therapies that address the underlying mechanisms of ALS. These efforts include gene therapy, RNA-based treatments, and neuroprotective agents. Based on recent Amyotrophic Lateral Sclerosis market growth
observations, investment in research pipelines is a key driver shaping the competitive landscape. Companies that successfully advance clinical candidates gain significant strategic advantages.
Another important growth factor is the availability of supportive regulatory frameworks. Orphan drug designations and accelerated approval programs enable faster market entry for ALS therapies. These policies not only encourage innovation but also improve patient access to new treatment options. As a result, manufacturers are increasingly prioritizing ALS within their rare disease portfolios.
Healthcare infrastructure improvements also support market growth. Advanced diagnostic tools allow earlier detection, while specialized care centers enhance treatment delivery. Additionally, expanding reimbursement coverage in certain regions improves affordability and access to therapies, further stimulating demand.
Get Full Reports:https://www.marketresearchfuture.com/reports/amyotrophic-lateral-sclerosis-market-5822
Technology adoption is strengthening market expansion as well. Digital platforms enable better patient monitoring, while data-driven research improves treatment personalization. These advancements help optimize care outcomes and improve long-term treatment adherence.
In summary, the Amyotrophic Lateral Sclerosis Market is driven by scientific progress, regulatory incentives, and growing healthcare capabilities. These combined factors create a stable foundation for continued market development.
FAQ
Q1: What is the main driver of ALS market growth?
A1: Increased R&D investment and regulatory support for rare diseases.
Q2: Does early diagnosis impact market growth?
A2: Yes, early diagnosis increases treatment demand and duration.
0 Comentários
0 Compartilhamentos
207 Visualizações
0 Anterior